The NuRD complex, which contains both the CHD4 ATPase and the HDAC2 deacetylase, is required for cells to repair and survive IR-induced DNA damage. Importantly, expression of the MTA1 sub-unit of NuRD is induced under hypoxic conditions, 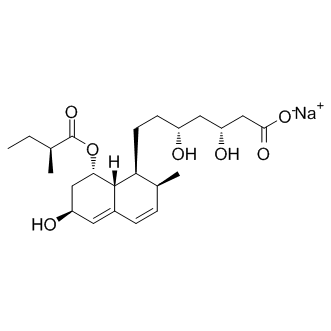 and both MTA1 and histone deacetylases contribute to Hif1a SCH727965 stability. This indicates that components of the NuRD complex may be upregulated by Hif1a. Therefore, we examined if components of the NuRD complex were increased by hypoxia mimics and if this induction contributed to the observed radioprotection by DMOG. To examine the transcriptional activity of Hif1a, we first reduced expression of Hif1a using shRNA. shRNA targeting Hif1a blocked the accumulation of Hif1a after treatment with either DMOG or CoCl2and reduced the levels of Hif1a mRNA. Silencing of Hif1a also inhibited the accumulation of VEGF mRNA, a key target of Hif1a, demonstrating that Hif1a function is abolished in the shRNAHif1a cells. Next, we determined if Hif1a regulates expression of the CHD4 and MTA3 genes. Figure 2D and 2E demonstrate that activation of Hif1a by CoCl2 increased the levels of CHD4 and MTA3 mRNA, and this increase was abolished when Hif1a was silenced with shRNA. This demonstrates that Hif1a is required for the accumulation of CHD4 and MTA3 mRNA after exposure to CoCl2. Further, CoCl2 increased the levels of Hif1a, CHD4 and MTA3 protein with similar kinetics, consistent with the Hif1adependent increase in their mRNA levels. Significantly, suppression of Hif1a with shRNA also reduced the basal levels of CHD4 protein, whereas basal levels of MTA3 were unaffected by loss of Hif1a. Further, loss of Hif1a greatly attenuated the accumulation of CHD4 after exposure to CoCl2, but had only a small impact on the accumulation of MTA3 protein. Figure 2 therefore demonstrates that increased levels of Hif1a lead to increased levels of MTA3 and CHD4 mRNA, potentially identifying CHD4 and MTA3 as transcriptional targets for Hif1a. Significantly, loss of Hif1a decreased both the basal and stimulated levels of CHD4 protein, indicating that Hif1a plays a critical role in maintaining basal and stimulated levels of CHD4. Previous work indicates that CHD4 can participate in the cells response to IR-induced DNA damage. This suggests that, because cells expressing shRNA to Hif1a have decreased levels of CHD4, they should be more sensitive to IR. Figure 3A demonstrates that cells lacking Hif1a exhibit a small but significant increase in radiosensitivity, consistent with a key role for Hif1a in regulating radiosensitivity. However, when shRNA was used to deplete CHD4 protein levels to a level similar to those detected in Hif1a depleted cellsno significant impact on radiosensitivity was seen. Similarly, silencing MTA3 expression with shRNA did not alter cellular radiosensitivity. We interpret this to mean that, while Hif1a contributes to cell survival after exposure to IR, this regulation of radiosensitivity is not mediated Adriamycin through the ability of Hif1a to regulate the expression of either CHD4 or MTA3. Therefore, although we have identified CHD4 and MTA3 as potential transcriptional targets for Hif1a, the ability of Hif1a to protect cells from radiation damage does not require either CHD4 or MTA3. It is more likely that upregulating CHD4 and MTA3, which are components of NuRD deacetylase complex, plays a key role in other processes, such as transcriptional repression, which are a feature of the hypoxia response. To further explore how stabilization of Hif1a regulates radiosensitivity, we examined a second group of proteins, the histone demethylases, which are transcriptionally activated by Hif1a.
and both MTA1 and histone deacetylases contribute to Hif1a SCH727965 stability. This indicates that components of the NuRD complex may be upregulated by Hif1a. Therefore, we examined if components of the NuRD complex were increased by hypoxia mimics and if this induction contributed to the observed radioprotection by DMOG. To examine the transcriptional activity of Hif1a, we first reduced expression of Hif1a using shRNA. shRNA targeting Hif1a blocked the accumulation of Hif1a after treatment with either DMOG or CoCl2and reduced the levels of Hif1a mRNA. Silencing of Hif1a also inhibited the accumulation of VEGF mRNA, a key target of Hif1a, demonstrating that Hif1a function is abolished in the shRNAHif1a cells. Next, we determined if Hif1a regulates expression of the CHD4 and MTA3 genes. Figure 2D and 2E demonstrate that activation of Hif1a by CoCl2 increased the levels of CHD4 and MTA3 mRNA, and this increase was abolished when Hif1a was silenced with shRNA. This demonstrates that Hif1a is required for the accumulation of CHD4 and MTA3 mRNA after exposure to CoCl2. Further, CoCl2 increased the levels of Hif1a, CHD4 and MTA3 protein with similar kinetics, consistent with the Hif1adependent increase in their mRNA levels. Significantly, suppression of Hif1a with shRNA also reduced the basal levels of CHD4 protein, whereas basal levels of MTA3 were unaffected by loss of Hif1a. Further, loss of Hif1a greatly attenuated the accumulation of CHD4 after exposure to CoCl2, but had only a small impact on the accumulation of MTA3 protein. Figure 2 therefore demonstrates that increased levels of Hif1a lead to increased levels of MTA3 and CHD4 mRNA, potentially identifying CHD4 and MTA3 as transcriptional targets for Hif1a. Significantly, loss of Hif1a decreased both the basal and stimulated levels of CHD4 protein, indicating that Hif1a plays a critical role in maintaining basal and stimulated levels of CHD4. Previous work indicates that CHD4 can participate in the cells response to IR-induced DNA damage. This suggests that, because cells expressing shRNA to Hif1a have decreased levels of CHD4, they should be more sensitive to IR. Figure 3A demonstrates that cells lacking Hif1a exhibit a small but significant increase in radiosensitivity, consistent with a key role for Hif1a in regulating radiosensitivity. However, when shRNA was used to deplete CHD4 protein levels to a level similar to those detected in Hif1a depleted cellsno significant impact on radiosensitivity was seen. Similarly, silencing MTA3 expression with shRNA did not alter cellular radiosensitivity. We interpret this to mean that, while Hif1a contributes to cell survival after exposure to IR, this regulation of radiosensitivity is not mediated Adriamycin through the ability of Hif1a to regulate the expression of either CHD4 or MTA3. Therefore, although we have identified CHD4 and MTA3 as potential transcriptional targets for Hif1a, the ability of Hif1a to protect cells from radiation damage does not require either CHD4 or MTA3. It is more likely that upregulating CHD4 and MTA3, which are components of NuRD deacetylase complex, plays a key role in other processes, such as transcriptional repression, which are a feature of the hypoxia response. To further explore how stabilization of Hif1a regulates radiosensitivity, we examined a second group of proteins, the histone demethylases, which are transcriptionally activated by Hif1a.
Author: targets inhibitor
The development and use of an ATB-BMPA based assay for labeling of the cytoplasmic glucose
While it has been generally assumed that all PIs possess the same degree of isoform selectivity as indinavir, direct comparisons of glucose transport blockade in GLUT1 versus GLUT4 expressing cells have been lacking. The binding affinityof indinavir for GLUT4 in the oocyte systemdiffers from that observed in primary adipocytes. While the basis for this difference is unknown, contributing factors may include subtle structural differences in the expressed transporter due to lipid composition, assay temperature, the presence of additional proteins, or other factors. It was therefore necessary to directly compare the ability of both first generation and newer PIs to alter GLUT1 versus GLUT4 activity. These data provide a more comprehensive assessment of similarities and differences in the behavior of these PIs on facilitative glucose transport. Several observations related to the ability of PIs examined in this study to compete for endofacial ATB BMPA binding have direct relevance to understanding the metabolic toxicities of these drugs in antiretroviral treatment regimens. Importantly, few studies to date have directly assessed the relationship between EX 527 intracellular PI concentrations and impaired glucose uptake. Whether PI import occurs via simple diffusion or through mediated transport, sufficient drug levels may be present within the cytosol even when serum levels are low. In addition, while it has been assumed that all PIs possess the same degree of GLUT isoform selectivity as indinavir, several PIs including ritonavir do not appear to distinguish among these transporters. Thus, the effects of some PIs on glucose homeostasis in tissues that do not express GLUT4may still be mediated by changes in glucose transport. Comparison of the effects of various PIs in these tissues may provide further insight into the mechanistic basis for altered glucose homeostasis. More comprehensive assessment of the ability of individual PIs to block each of the other known GLUTs may provide insight into glucotoxicities. While atazanavir has a more favorable metabolic profile relative to first generation PIs, the current studies demonstrate that at drug levels above those typically CUDC-907 achieved during clinical use, the potential for significantly altering glucose transport exists. The inability of tipranavir to alter either ATB BMPA binding or 2DG transport further supports the role of peptidomimetic structure in mediating binding to GLUTs. Understanding of the molecular basis for the development of insulin resistance in HIV infected patients treated with PIs has already contributed to success in developing drugs within this class that do not directly alter glucose homeostasis. Nevertheless, many of these newer agents including tipranavir are associated with dyslipidemiaand may therefore indirectly contribute to impaired insulin signaling. Furthermore, with the potential for development of viral resistance over time, the need for continued drug development remains. Characterization of the molecular interactions between candidate drugs and GLUTs will assist ongoing efforts for rationale drug design, not only for antiviral efficacy, but also for metabolic toxicity. Beyond further understanding of the mechanisms for PImediated insulin resistance, the ability to distinguish compounds that selectively interact with GLUT4 from those that bind to both GLUT1 and GLUT4 suggests that it may be possible to identify small molecule inhibitors of each of the other known GLUTs. The availability of specific pharmacologic inhibitors of these 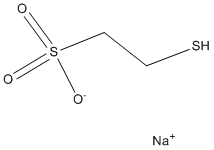 transporters would provide a means to further characterize the functional role of these isoforms prior to the induction of potential compensatory changes in gene knockout models.
transporters would provide a means to further characterize the functional role of these isoforms prior to the induction of potential compensatory changes in gene knockout models.
Notably asiatic acid had the highest water solubility of the entire series which in this case
ABHD12 has remained a challenging target for inhibitor development as there are no crystal structures available, number of known inhibitors is low and the existing MLN4924 side effects activity data are GDC-0879 limited. In order to find novel lead structures for selective inhibitors of recently discovered serine hydrolases, exploring the activity of natural compounds may offer valuable information for this developing process. For instance, plant-derived pentacyclic triterpenes such as betulinic, oleanolic and ursolic acid are interesting molecules as they all are bioactive and widespread in nature and their therapeutic potential is well documented see also reviews and references cited therein. In addition, their multi-targeting biological activity, low toxicity, easy availability, and core structure offering good starting point for chemical modifications, make triterpenoids appealing source for the drug discovery. Along this line, recent studies have revealed that triterpenes may include potential candidates for novel inhibitors of e.g. endocannabinoid hydrolases. Indeed, pristimerin has been shown to inhibit MAGL activity in in vitro studies. In another study, a mixture of a/b-amyrin was shown to reduce inflammatory and neuropathic hyperalgesia in mice through activation of the cannabinoid CB1 and CB2 receptors. Interestingly, despite their high affinity towards CB1R, the compounds failed to show any cannabimimetic effects in the tetrad test. In addition, a- and b-amyrin were reported to inhibit 2-AG-hydrolysis in pig brain homogenates. The molecular target of this action was not identified. Our preliminary screening efforts to identify novel serine hydrolase inhibitors among various chemical compounds revealed unexpectedly that ursolic acid was able to selectively inhibit ABHD12 with negligible effect on ABHD6 or MAGL activity. Inspired by this finding, we selected various commercial triterpenes/triterpenoids as well as recently reported betulin-based triterpenes for further evaluation. In this paper, we report the inhibitory activity of these compounds towards human ABHD12. Based on the activity data we have established preliminary structure-activity relationships and constructed the first pharmacophore model for betulin-based triterpenes. This model should prove useful in the discovery of novel lead structures for ABHD12 selective inhibitors. Although the triterpenoids typically interact with multiple protein targets, we witnessed unprecedented selectivity towards ABHD12 among the metabolic serine hydrolases, as activity-based protein profiling of mouse brain membrane proteome indicated that the representative ABHD12 inhibitors did not inhibit other serine hydrolases, nor did they target cannabinoid receptors. Pentacyclic triterpenes can be classified into three different groups: lupanes, oleananes and ursanes. Derivatives of triterpenes are called triterpenoids. In this study, commercially available triterpenes 1�C11 and triterpenoids 12�C15 were purchased from different chemical vendors and tested for their ability to inhibit hydrolase activity in lysates of HEK293 cells transiently overexpressing human ABHD12. The inhibition data are presented in Table 1. In the lupane series, an importance of a carboxyl group at position 17 was shown as betulinic acid had the highest inhibitory activity. However, lipophilicity differences should also be taken into consideration as the compound with the lowest logD also had the highest inhibitory activity. In the ursane series, similar effect of the carboxyl 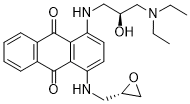 group at position 17 was observed as ursolic acid showed higher inhibition activity compared to a-amyrin that has a methyl group at this position. Asiatic acid, which has a primary hydroxyl group at the position 4, was completely devoid of activity, demonstrating the importance of this position for hABHD12 inhibition.
group at position 17 was observed as ursolic acid showed higher inhibition activity compared to a-amyrin that has a methyl group at this position. Asiatic acid, which has a primary hydroxyl group at the position 4, was completely devoid of activity, demonstrating the importance of this position for hABHD12 inhibition.
An endothelium-derived relaxing factor plays an important physiologic role in the control of vascular tone
Although the effects of eNOS-derived NO on 26S proteasome functionality are not completely elucidated, the effect of NO on proteasome has drawn increased attention. NO has been found to inhibit the 26S proteasome, resulting in diminished p53 degradation or loss of cell viability. The suppressive effect is PF-4217903 956905-27-4 mediated by S-nitrosylation and reduced proteasomal subunit expression in vascular smooth muscle cells. However, others report that NO enhances proteasome activity and that the activation promoted by NO donor is thought to reduce neutral ceramidase or to protect the endothelial cell from damage induced by H2O2. It is unclear how to reconcile these discrepancies. One plausible solution could be testing the NO-exerted effects in an appropriate 26S proteasome reporter system in intact cells. The 26S proteasome functionality can be regulated through mechanisms dependent and/or independent of proteasomal protease-like activities. Until the first report of imaging 26S proteasome in living cells with a reporter system expressing UbG76V-GFP, assessments of 26S proteasome functionality have predominantly relied on the assay of protease-like activities in whole cell lysates or purified 26S proteasomes. UbG76V-GFP was engineered by expressing a surrogate protein substrate fused with a GDC-0879 Raf inhibitor ubiquitin mutant. The UbG76V mutation is crucial because it makes this GFP-bound ubiquitin resistant to removal by deubiquitinase, which would otherwise prevent the GFP from recognition and degradation by 26S proteasomes. Thus, the UbG76V mutation makes the UbG76V-GFP protein a perfect 26S proteasome substrate. As such, protein levels of GFP represent 26S proteasome functionality in cells. As a surrogate proteasome substrate, UbG76V-GFP was initially designed for the assessment of 26S proteasome inhibition in intact cells or mice. This is because when the 26S proteasome is sufficiently suppressed, the otherwise degraded poly Ub-GFP would accumulate to a significant level for quantification of the GFP fluorescence. Accordingly, UbG76V-GFP mice have been used to monitor proteasome inhibition in models of amyotrophic lateral sclerosis, Alzheimer’s disease, and polyglutamine diseases. By taking the advantage of the UbG76V-GFP reporter, together with an additional modification to its detection, we have been able to monitor the enhancement of 26S proteasome functionality in early diabetes and in glucosetreated cultured cells. This has been accomplished by employing a more sensitive approach that has combined ubiquitin enrichment through ubiquitin affinity binding purification followed by Western blotting of the GFP proteins. Physiological regulation of 26S proteasomes are complex which mechanisms remain incompletely understood; however, it is believed that the mechanisms are multifaceted and include posttranslational modifications. O-GlcNAc modification was the first endogenous inhibitor of the 26S proteasome identified in cells, although the physiological relevance has yet to be established. By utilizing the 26S proteasome reporter system both in cultured cells and mice, the present study sought to identify NO, particularly the eNOS-derived, as an endogenous regulator of the 26S proteasome in vascular endothelial cells and the involvement of proteasome O-GlcNAcylation. With a 26S proteasome reporter system, this study has identified a new mechanism by which NO affects 26S proteasome functionality in vascular endothelial cells. The presented evidence supports an alternative pathway where eNOS-derived NO blocks  26S proteasome functionality through OGT, the essential enzyme that upregulates protein O-GlcNAc modification. Mechanistically, like NO donors, the eNOS-generated NO increased an OGT-dependent O-GlcNAc modification, likely of Rpt2, one of the subunit of the proteasome regulatory.
26S proteasome functionality through OGT, the essential enzyme that upregulates protein O-GlcNAc modification. Mechanistically, like NO donors, the eNOS-generated NO increased an OGT-dependent O-GlcNAc modification, likely of Rpt2, one of the subunit of the proteasome regulatory.
Demonstrated that FITC-YARA uptake of the MK2-inhibitor peptides was investigated
Polyacrylamide gels were chosen as the model substrate for this VE-821 experiment because stiffness can be modulated by changing the percentage of bisacrylamide crosslinker within the system. Additionally, polyacrylamide gels are clear, non-fluorescent, and have the ability to covalently link proteins to the surface. Unlike most other systems, polyacrylamide gels are inert to protein adsorption and cell adhesion; thus, cellular adhesion can be controlled by functionalizing the gels with an extracellular matrix protein. The adhesion of cells to the gel is then solely attributed to cellular binding to the ECM protein. To confirm that uptake occurs through similar mechanisms on the soft polyacrylamide gel compared to tissue culture plastic, we characterized the mechanism of uptake by treating the cells with FITC-peptide at 4uC to determine if uptake is PF-4217903 956905-27-4 energydependent and with a pharmacological inhibitor of caveolae-mediated endocytosis, methyl-b-cyclodextrin. Figure 7A and B shows that uptake of FITC-YARA on soft substrates is inhibited at low temperatures and is inhibited with MbCD pretreatment, similar to what has been observed on tissue culture plastic. In addition, we were interested in evaluating uptake as a function of initial seeding density. As shown in Figure 8, seeding density does change the uptake of FITC-YARA. A high initial cell seeding density decreased the amount of FITC-YARA that was endocytosed by mesothelial cells seeded on soft substrates compared to tissue culture plastic. This was completely opposite of what was observed at a lower cell density and agreed with functional results: increased cell density decreased uptake of peptide and efficacy of cytokine suppression. Because cells cultured on tissue culture polystyrene showed more pronounced actin stress fibers than those grown on polyacrylamide substrates, we evaluated the effect of actin filaments on YARA uptake using flow cytometry. Using LPA, we induced actin filament formation and using cytochalasin D we disrupted actin filament formation. While cells treated with peptide showed an increased fluorescent signal, indicative of peptide uptake, as compared to untreated cells, treatment with LPA, or treatment with cytochalasin D, had no effect on peptide uptake. This data suggests that peptide uptake is not affected by actin polymerization. Endosome trafficking is dependent on microtubules, thus, nocodazole was used to interfere with microtubule polymerization to evaluate the effects of microtubules on peptide uptake. Cells treated with nocodazole showed a pronounced increase in YARA uptake, in a dose dependent manner, as compared to untreated cells. This data confirms the importance of microtubules in YARA uptake or trafficking. In this paper, we provide evidence that matrix stiffness regulates intracellular uptake of MK2-inhibitor peptides. Not only is there an increase in uptake as observed by flow cytometry, but there is a functional difference as characterized by the production of proinflammatory cytokines. Based on the quantification of surface fibronectin protein, which showed equivalent amounts of ECM protein between the soft and stiff polyacrylamide gel, the difference in YARA uptake can be attributed directly to the effect of stiffness. Characterization of the fibronectin protein is important because several investigators have demonstrated that cells respond morphologically to varying amounts of matrix proteins. We wanted to ensure that the cell response observed could be appropriately attributed to matrix stiffness and not the matrix proteins. Importantly, the mechanism of uptake of FITC-YARA does not appear to change when cells are seeded  on soft substrates. Mesothelial cells seeded on tissue culture plastic show inhibition of uptake at 4uC and with the removal of cholesterol using the pharmacological inhibitor, MbCD.
on soft substrates. Mesothelial cells seeded on tissue culture plastic show inhibition of uptake at 4uC and with the removal of cholesterol using the pharmacological inhibitor, MbCD.