The present data would add the kidney as a direct sensor organ that may interfere in phosphate homeostasis via its own FGF23 production. It is noteworthy that the FGF23 co-receptor CPI-613 Klotho is expressed weakly in the proximal tubules -despite the fact that the FGF23 phosphaturic action is substantially exerted at this levelwhereas distal tubules showed more abundant Klotho expression. Two mechanisms have been proposed : either FGF23 acts on proximal tubules via FGFR-Klotho signaling to directly regulate NaPi co-transporters, and/or acts on the distal tubules to induce a paracrine signal to proximal tubules. The putative paracrine factor is the secreted Klotho Crizotinib itself able to directly inhibit NaPi co-transporters and to activate several ion channels in proximal tubules. The significance of the local production of FGF23 is obscure. FGF23 expression might represent an adaptive response to early injury as a mechanism by which the ZDF rat kidney could maintain phosphate homeostasis. Indeed, at 4 months, Klotho was normally expressed in the kidney allowing FGF23 phosphaturic activity to maintain serum phosphate levels similar to those of lean rats. Finding that ZDF rats exhibited a 2fold higher urinary phosphorus excretion than lean rats could also be attributed to a 2-fold increased food intake in ZDF rats. As renal disease progressed, despite the increasing FGF23 levels, fractional phosphorus excretion decreased possibly due to a lower renal Klotho expression and a reduction in functioning nephrons, with the net result of increased serum phosphate levels. Our finding of decreased Klotho expression in the kidney of ZDF 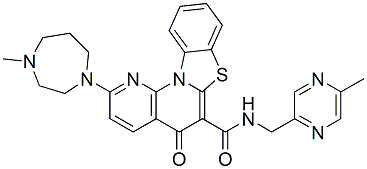 rats during overt nephropathy is in agreement with findings of decline of renal Klotho both in rodent models of chronic renal damage and patients with CKD. Klotho is a putative aging suppressor, and there is compelling evidence that its depletion is associated with oxidative stress, inflammation, accelerated aging and renal fibrosis. An important observation of the present study is that ramipril therapy, which limited proteinuria and renal damage in ZDF rats, effectively prevented the time-dependent increase of renal FGF23 expression, and almost normalized Klotho expression. A cross talk between the renin-angiotensin-system and Klotho-FGF23 axis has been suggested. Angiotensin II causes renal Klotho loss leading to disrupted FGF23 signaling and reduced phosphaturia. In a model of renal damage characterized by renal RAS activation and Klotho downregulation, the treatment with an angiotensin II type 1 receptor blocker prevented the loss of Klotho expression and ameliorated renal histology. Here, ramipril by recovering renal Klotho expression allowed re-engagement of serum and residual renal FGF23 to exert phosphaturic activity, resulting in normalization of serum phosphate levels in diabeticrats. Ramipril was capable to restore the mRNA expression of NaPi-2a co-transporter to normal levels, which however, did not translate into recovery of the protein on the brush border of proximal tubules. Discrepancy between NaPi-2a co-transporter mRNA and protein expression could be attributable to post-transcriptional events that precluded a full restoration of the protein. This would explain the high level of phosphorus excretion at 8 months in ramipril-treated diabetic rats. In early stage of CKD, elevation of FGF23 represents an appropriate physiological response to prevent hyperphosphatemia. However, with CKD progression, the excess of biologically active FGF23 becomes no longer protective and may instead lead to pathological off-target effects. Elevated circulating levels of FGF23 were associated with vascular dysfunction, atherosclerotic burden and left ventricular hyperthrophy in CKD patients, and FGF23 directly induced hypertrophy of cultured cardiomyocytes. Recently, inflammation has been identified as another potential off-target, in that higher FGF23 levels were independently associated with higher levels of inflammatory markers in patients with CKD. Consistently, here, in ZDF rats the reduction of renal FGF23 production after treatment with ACE inhibitor was paralleled by less infiltrates of inflammatory cells in the renal interstitium. To maximize renoprotection in ZDF rats one could foresee adding other compounds to the ACE inhibitor. In the search for an effective therapy, molecules that have the ability to limit, but not to abrogate FGF23 production would represent a valuable approach. A recent study has shown vascular calcification associated with increased risk of mortality in CKD rats.
rats during overt nephropathy is in agreement with findings of decline of renal Klotho both in rodent models of chronic renal damage and patients with CKD. Klotho is a putative aging suppressor, and there is compelling evidence that its depletion is associated with oxidative stress, inflammation, accelerated aging and renal fibrosis. An important observation of the present study is that ramipril therapy, which limited proteinuria and renal damage in ZDF rats, effectively prevented the time-dependent increase of renal FGF23 expression, and almost normalized Klotho expression. A cross talk between the renin-angiotensin-system and Klotho-FGF23 axis has been suggested. Angiotensin II causes renal Klotho loss leading to disrupted FGF23 signaling and reduced phosphaturia. In a model of renal damage characterized by renal RAS activation and Klotho downregulation, the treatment with an angiotensin II type 1 receptor blocker prevented the loss of Klotho expression and ameliorated renal histology. Here, ramipril by recovering renal Klotho expression allowed re-engagement of serum and residual renal FGF23 to exert phosphaturic activity, resulting in normalization of serum phosphate levels in diabeticrats. Ramipril was capable to restore the mRNA expression of NaPi-2a co-transporter to normal levels, which however, did not translate into recovery of the protein on the brush border of proximal tubules. Discrepancy between NaPi-2a co-transporter mRNA and protein expression could be attributable to post-transcriptional events that precluded a full restoration of the protein. This would explain the high level of phosphorus excretion at 8 months in ramipril-treated diabetic rats. In early stage of CKD, elevation of FGF23 represents an appropriate physiological response to prevent hyperphosphatemia. However, with CKD progression, the excess of biologically active FGF23 becomes no longer protective and may instead lead to pathological off-target effects. Elevated circulating levels of FGF23 were associated with vascular dysfunction, atherosclerotic burden and left ventricular hyperthrophy in CKD patients, and FGF23 directly induced hypertrophy of cultured cardiomyocytes. Recently, inflammation has been identified as another potential off-target, in that higher FGF23 levels were independently associated with higher levels of inflammatory markers in patients with CKD. Consistently, here, in ZDF rats the reduction of renal FGF23 production after treatment with ACE inhibitor was paralleled by less infiltrates of inflammatory cells in the renal interstitium. To maximize renoprotection in ZDF rats one could foresee adding other compounds to the ACE inhibitor. In the search for an effective therapy, molecules that have the ability to limit, but not to abrogate FGF23 production would represent a valuable approach. A recent study has shown vascular calcification associated with increased risk of mortality in CKD rats.
Author: targets inhibitor
In combination with lowdose CNI was associated with low acute rejection rate
In terms of ERL, Simone et al recently reported that ERL and particularly good renal function. However, in another study, the use of combination CsA and mTORis leaded to potential 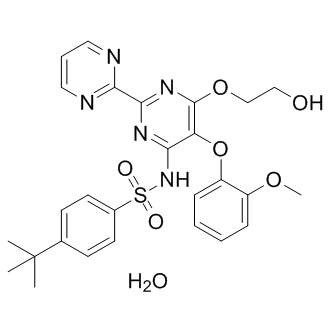 longterm CNI nephrotoxicity. Since the number of SRL-based and ERL-based RCTs included in this meta-analysis is small, more high-quality RCTs based on SRL and ERL should be conducted to draw a clear conclusion on whether mTORis-based CNI minimization protocols are effective and safe in patients with impaired renal function. However, according to the results from the meta-analysis of observational trials and considering their well known anti-tumor effects, mTORis may be a good alternative for MMF to reduce or replace CNI in liver transplant recipients with a pre-transplant diagnosis of hepatocellular carcinomaand post-transplant renal dysfunction. However, clinicians should pay attention to the increased risks of infections when SRL is used. Undoubtedly, there are some limitations in the current metaanalysis as others. Firstly, we included studies using different regimens without comparing between themselves, it make us difficult to figure out which combination is the best one although the current data show that the MMF-based CNI minimization protocol received the greatest supports. Secondly, most of the studies we included didn’t undertake follow-ups longer than 12 months, giving us insufficient data on how CNI minimization would affect long-term graft or patient survival. Finally, as shown in Table 2, the risk of bias of the included randomized trials was relatively high, since no study was double blind designed and only 3 of 10 studies conducted intention-to-treat analysis, which may attenuate the power of the current study. In conclusion, this meta-analysis included all current relevant studies from various countries covering different populations. It can make up to the shortage of small sample size and limited population of individual studies, providing stronger evidence on the clinical Enzalutamide application of CNI minimization protocols. It is convincing that CNI minimization can improve renal function in liver transplant patients with CNI-related renal impairment, while has an equal or similar effect on acute rejection and patient survival as routine CNI regimen. However, it should be cautious to use SRL-based minimization regimens in patients with high risks of infections. Studies in the future should try to figure out whether this improved renal function can prolong long-term patient or graft survival, and which minimization protocol is the standard one in various Z-VAD-FMK combinations. The development and clinical use of HIV protease inhibitors has greatly contributed to the transition of HIV infection from a once fatal disease to its current status as a chronic condition. Tempering enthusiasm for this major advance in HIV treatment is the growing realization that patients treated with combined antiretroviral treatment regimens are at increased risk for the development of pro-atherogenic metabolic side effects including dyslipidemia and insulin resistance. A direct contribution of HIV protease inhibitors to altered glucose homeostasis has been established from several clinical studies. Despite growing awareness of these treatment-related side effects, understanding the mechanisms leading to the development of insulin resistance in treated HIV infection remains incomplete. The ability of PIs to induce insulin resistance in treated patients is not shared by all agents within this drug class. Indinavir and ritonavir appear to have the greatest effect on glucose transport both in vitro and in vivo whereas newer PIs such atazanavir and tipranavir have minimal to no effect on insulin sensitivity.
longterm CNI nephrotoxicity. Since the number of SRL-based and ERL-based RCTs included in this meta-analysis is small, more high-quality RCTs based on SRL and ERL should be conducted to draw a clear conclusion on whether mTORis-based CNI minimization protocols are effective and safe in patients with impaired renal function. However, according to the results from the meta-analysis of observational trials and considering their well known anti-tumor effects, mTORis may be a good alternative for MMF to reduce or replace CNI in liver transplant recipients with a pre-transplant diagnosis of hepatocellular carcinomaand post-transplant renal dysfunction. However, clinicians should pay attention to the increased risks of infections when SRL is used. Undoubtedly, there are some limitations in the current metaanalysis as others. Firstly, we included studies using different regimens without comparing between themselves, it make us difficult to figure out which combination is the best one although the current data show that the MMF-based CNI minimization protocol received the greatest supports. Secondly, most of the studies we included didn’t undertake follow-ups longer than 12 months, giving us insufficient data on how CNI minimization would affect long-term graft or patient survival. Finally, as shown in Table 2, the risk of bias of the included randomized trials was relatively high, since no study was double blind designed and only 3 of 10 studies conducted intention-to-treat analysis, which may attenuate the power of the current study. In conclusion, this meta-analysis included all current relevant studies from various countries covering different populations. It can make up to the shortage of small sample size and limited population of individual studies, providing stronger evidence on the clinical Enzalutamide application of CNI minimization protocols. It is convincing that CNI minimization can improve renal function in liver transplant patients with CNI-related renal impairment, while has an equal or similar effect on acute rejection and patient survival as routine CNI regimen. However, it should be cautious to use SRL-based minimization regimens in patients with high risks of infections. Studies in the future should try to figure out whether this improved renal function can prolong long-term patient or graft survival, and which minimization protocol is the standard one in various Z-VAD-FMK combinations. The development and clinical use of HIV protease inhibitors has greatly contributed to the transition of HIV infection from a once fatal disease to its current status as a chronic condition. Tempering enthusiasm for this major advance in HIV treatment is the growing realization that patients treated with combined antiretroviral treatment regimens are at increased risk for the development of pro-atherogenic metabolic side effects including dyslipidemia and insulin resistance. A direct contribution of HIV protease inhibitors to altered glucose homeostasis has been established from several clinical studies. Despite growing awareness of these treatment-related side effects, understanding the mechanisms leading to the development of insulin resistance in treated HIV infection remains incomplete. The ability of PIs to induce insulin resistance in treated patients is not shared by all agents within this drug class. Indinavir and ritonavir appear to have the greatest effect on glucose transport both in vitro and in vivo whereas newer PIs such atazanavir and tipranavir have minimal to no effect on insulin sensitivity.
Injection of thiamet-G led to decreased phosphorylation but increased tau phosphorylation at Ser199
We and other groups have previously shown that OGlcNAcylation regulates phosphorylation of tau in a reciprocal manner overall. Our observations of decreased tau phosphorylation at Thr181, Thr212, Ser214, Ser262/Ser356, Ser404 and Ser409 are consistent with previous studies, further supporting the reciprocal relationship between O-GlcNAcylation and phosphorylation of tau at these sites. To understand the underlying mechanism by which FDA-approved Compound Library thiamet-G increased tau phosphorylation at some other phosphorylation sites in the mouse brain, we investigated the effects of thiamet-G injection on the major tau kinases. We found that thiamet-G at the dose injected into the brain completely blocked Ser9 phosphorylation of GSK3b, indicating a marked activation of this major tau kinase. Our previous studies have demonstrated that tau phosphorylation at Ser199, Ser202 and Ser396 are mainly regulated by GSK-3b. Taken together, we conclude that the elevation of tau phosphorylation at Ser199, Ser202, Ser396 and Ser422 results from the combinational effects of the increase in tau O-GlcNAcylation and the activation of GSK-3b activity, and that GSK-3b activation predominated and led to the net increase in tau phosphorylation at these sites in the mouse brain. Our results showed that GSK-3b was not activated in cultured neuronal cells treated with thiametG, consistent with the absence of any increase in tau phosphorylation at these phosphorylation sites. In a previous study, when thiamet-G was administered to rats orally for 24 hrs, tau phosphorylation at these sites was not found to be increased. Whether the discrepancy between this previous study and the present study is due to different routes of drug administration, the attainment of different doses within the brain, or the use of different speciesis currently unknown. It is possible that there is either a dose-dependent effect of MK-2206 1032349-77-1 thiametG on 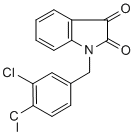 GSK-3b�Cstimulation or an off target effect of thiamet-G when used at high doses directly in the brain. Indeed, it is likely that the icv injection of this study led to a much higher thiamet-G concentration in the central nervous system than that from oral dosing. Unfortunately, GSK-3b modification and activity in the brain was not examined in the previous study, so direct comparisons are not possible. Our results indicated marked differences in the effects of thiamet-G on tau phosphorylation between the mouse brains and the cultured neurons. Further experiments indicated that thiametG�Cinduced increase of tau phosphorylation at several sites resulted from activation of GSK-3b, a major tau kinase, but this activation did not occur in cultured cells. Different regulations of tau phosphorylation by extracellular signaling between the brains and the cultured neurons might also contribute to the different results we observed. A previous study has demonstrated that tau phosphorylation is regulated by FGF-2 through GSK-3b. Our observations of GSK-3b activation in the brains but not in the cultured cells are consistent with this speculation. The mice we used for this study expressed the wild-type human tau isoform. Although we cannot absolutely rule out the possibility that the different results between the mouse brains and the cell cultures might also result from different tau species, this possibility is very small, because our observations that thiamet-G�Cinduced GSK-3b activation in the brains, but not in the cultured cells, can explain the different results in these two systems nicely. Our studies on the upstream regulating kinases of GSK-3b suggest that thiamet-G led to marked GSK-3b activation.
GSK-3b�Cstimulation or an off target effect of thiamet-G when used at high doses directly in the brain. Indeed, it is likely that the icv injection of this study led to a much higher thiamet-G concentration in the central nervous system than that from oral dosing. Unfortunately, GSK-3b modification and activity in the brain was not examined in the previous study, so direct comparisons are not possible. Our results indicated marked differences in the effects of thiamet-G on tau phosphorylation between the mouse brains and the cultured neurons. Further experiments indicated that thiametG�Cinduced increase of tau phosphorylation at several sites resulted from activation of GSK-3b, a major tau kinase, but this activation did not occur in cultured cells. Different regulations of tau phosphorylation by extracellular signaling between the brains and the cultured neurons might also contribute to the different results we observed. A previous study has demonstrated that tau phosphorylation is regulated by FGF-2 through GSK-3b. Our observations of GSK-3b activation in the brains but not in the cultured cells are consistent with this speculation. The mice we used for this study expressed the wild-type human tau isoform. Although we cannot absolutely rule out the possibility that the different results between the mouse brains and the cell cultures might also result from different tau species, this possibility is very small, because our observations that thiamet-G�Cinduced GSK-3b activation in the brains, but not in the cultured cells, can explain the different results in these two systems nicely. Our studies on the upstream regulating kinases of GSK-3b suggest that thiamet-G led to marked GSK-3b activation.
Identification of these resistance conferring mutations by the formation of additional stabilizing H-bonds and hydrophobic contacts
Figure 10 summarizes the key conclusions from the preliminary studyand this current investigation. Amino acids that are critical for HER2ligand interaction include Lys724, Lys736, and Cys805. As illustrated in Figure 10, binding at the key amino acids results in blocking of the ATP binding site entrance, and may result in inhibition of HER2 activity. Analysis of the candidates indicates that carbonyl, carboxylic acid, and hydroxyl groups are critical moieties for stable binding. Based on the results of this study, the natural compound candidates have potential as biologically active compounds with improved stability in HER2. Designing HER2 inhibitors with carbonyl, carboxyl, and hydroxyl groups available for H-bond formation may improve protein-ligand stability. Mitotic kinases play crucial roles in regulation of cell division, yet aberrations in their expression and function are known to be involved in cancer initiation and progression. Targeting these kinases has proven in recent years to be an exciting avenue for alternative cancer therapies. The Aurora kinases have emerged as particularly promising targets due their roles in regulating multiple signalling pathways crucial for accurate cell division. Localization and function of each subtype �C Aurora A, B and C, has been studied and reviewed extensively in the recent literature. The association and implication of the Aurora kinases in cancer stems from early studies that revealed aberrant expression of both Aurora A and B in many solid and hematological malignancies. This association of Aurora kinase overexpression with a malignant phenotype has been functionally validated. Deregulation of the Aurora kinases disrupts mitotic processes crucial for accurate cell division leading to chromosomal instability and aneuploidyhowever a complete understanding of their role in tumourigenesis remains elusive. Reports of the role and function of Aurora A and B in leukaemia have been largely limited to expression studies in cell lines and small cohort clinical studies. Increased expression of Aurora A has been reported in many leukaemias, while the expression of Aurora B has shown no clear trend. Despite this, both Aurora A and B have been exploited as potential targets for therapeutic intervention. The promise of the Aurora kinases as anticancer targets has been such that small molecule inhibition as drug therapy is a rapidly Carfilzomib developing area of research. Early successful candidates in preclinical testing were pan-Aurora inhibitors such as VX-680, however it was shown that the dominant phenotype arising from these agents was that of Aurora B inhibition. Aurora B specific inhibitors such as AZD1152have since shown increasing promise and have reached early stage clinical trials against both solid and haematological malignancies. The earliest documented Aurora 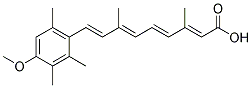 B inhibitor ZM447439 has also been well characterised as a probe of the cellular biology of Aurora B. Cellular OTX015 phenotypes of these agents such as inhibition of histone H3 phosphorylation, cytokinesis failure, and polyploidisation are consistent with inhibition of Aurora B. As yet, however, the specific factors that will influence sensitivity and resistance to Aurora kinase inhibitors have not been adequately addressed. A major drawback of molecularly targeted agents is the likelihood of acquired clinical resistance. Early success of the BCR-ABL kinase targeting drug Imatinib in the treatment of chronic myelogenous leukaemia was followed by the rapid emergence of clinical resistance. Resistance was discovered to be mediated by point mutations in the kinase domain preventing drug binding but maintaining catalytic activity.
B inhibitor ZM447439 has also been well characterised as a probe of the cellular biology of Aurora B. Cellular OTX015 phenotypes of these agents such as inhibition of histone H3 phosphorylation, cytokinesis failure, and polyploidisation are consistent with inhibition of Aurora B. As yet, however, the specific factors that will influence sensitivity and resistance to Aurora kinase inhibitors have not been adequately addressed. A major drawback of molecularly targeted agents is the likelihood of acquired clinical resistance. Early success of the BCR-ABL kinase targeting drug Imatinib in the treatment of chronic myelogenous leukaemia was followed by the rapid emergence of clinical resistance. Resistance was discovered to be mediated by point mutations in the kinase domain preventing drug binding but maintaining catalytic activity.
Enhance the rational use of combinatorial regimens involving temsirolimus and PI3K inhibitors in temsirolimus and BEZ235
It has previously been shown that treatment with BEZ235 or ZSTK474 results in cell cycle arrest at G1. Our study demonstrates that cells were more likely to arrest in G1 if they had been treated with either BEZ235 or ZSTK474 with ICI 182780 temsirolimus compared to controls or single agent treatment. This may be attributed to the ability of BEZ235 to promote increased expression of the CDKI p27. Accordingly, we also detected elevated p27 expression when endometrial cancer cells were treated with BEZ235 alone or in combination with temsirolimus. While inhibition of prosurvival Akt signaling is cytotoxic, the mechanism of cell death involves autophagy and apoptosis. We observed a decrease in the autophagy marker, LC-3I, in response to dual mTOR/PI3K inhibition, implicating autophagy. Others have shown that depletion of all three Akt isoforms promoted tumor regression through initiation of autophagy, and inhibition of mTOR with the alkylphospholipid perifosine induces autophagic cell death. BEZ235 has also been shown to Niltubacin supply induce caspase-independent apoptosis in a mechanism that includes PARP cleavage, which we also observed in our study. Taken together, these data suggest that the mechanism of cell death is through autophagy and caspase-independent apoptosis. Molecular profiling of the endometrial cancer cell lines revealed that sensitivity toward drug treatment correlates with loss of PTEN expression and hyper-activation of Akt. In endometrial tumors, loss of PTEN has previously been shown to correlate with elevated Akt phosphorylation and results in poor outcomes. Our findings are consistent with earlier studies showing low expression of PTEN in RL95-2, AN3CA, ECC-1, and Ishikawa H cells and high expression of PTEN in Hec50, Hec1A, and KLE cells. Furthermore, RL95-2, AN3CA, ECC-1, and Ishikawa H cells harbor mutant PTEN, whereas KLE, Hec50, Hec1A, and Hec1Bcells express wildtype PTEN. The fact that Hec50 cells contain both wildtype PTEN and high Akt phosphorylation can be explained by recent data demonstrating that PI3KR1, a regulatory subunit of PI3K, is mutated in Hec50 cells and thus may phenocopy loss of PTEN. Additional investigation is necessary to understand why Ishikawa H cells, which have high Akt phosphorylation and a loss of active PTEN, are relatively resistant to  temsirolimus. These data highlight the fact that molecular profiling does not always predict for response due to the complexity of pathways governing tumor initiation and progression. Furthermore, basal Akt phosphorylation correlates with response to another rapalog, RAD001, in a panel of various cancer cell lines. Studies involving PTEN +/2 mice or cell lines devoid of PTEN show that PTEN-deficient tumors are sensitive to mTOR inhibition. A phase I clinical trial demonstrated that 63% of patients with PTEN-negative tumors displayed tumor regression when treated with drugs targeting the PI3K/Akt/ mTOR signaling pathway. These results are consistent with our data that cell lines with little or no PTEN are more sensitive to temsirolimus alone and with the combination of temsirolimus and BEZ235. In accord with our findings, single-agent BEZ235 has been shown to inhibit proliferation of endometrial cancer cells harboring PIK3CA and/or PTEN mutations by other investigators. These studies reported herein promote a further understanding of the potential underlying mechanisms of both primary cell resistance and the development of acquired resistance after therapy, both of which can be overcome with the combination of mTOR and PI3K inhibitors. Our data underscore the need to inhibit PI3K/Akt/mTOR signaling at multiple levels to achieve sustained cellular responses.
temsirolimus. These data highlight the fact that molecular profiling does not always predict for response due to the complexity of pathways governing tumor initiation and progression. Furthermore, basal Akt phosphorylation correlates with response to another rapalog, RAD001, in a panel of various cancer cell lines. Studies involving PTEN +/2 mice or cell lines devoid of PTEN show that PTEN-deficient tumors are sensitive to mTOR inhibition. A phase I clinical trial demonstrated that 63% of patients with PTEN-negative tumors displayed tumor regression when treated with drugs targeting the PI3K/Akt/ mTOR signaling pathway. These results are consistent with our data that cell lines with little or no PTEN are more sensitive to temsirolimus alone and with the combination of temsirolimus and BEZ235. In accord with our findings, single-agent BEZ235 has been shown to inhibit proliferation of endometrial cancer cells harboring PIK3CA and/or PTEN mutations by other investigators. These studies reported herein promote a further understanding of the potential underlying mechanisms of both primary cell resistance and the development of acquired resistance after therapy, both of which can be overcome with the combination of mTOR and PI3K inhibitors. Our data underscore the need to inhibit PI3K/Akt/mTOR signaling at multiple levels to achieve sustained cellular responses.