Our results show that bortezomib and paclitaxel combined treatment is able to target the TKIsresistant cell lines with the T315I mutation in Bcr-Abl. Collectively, our findings indicate that the bortezomib in combination with four different mitotic inhibitors, that repress mitosis by different mechanisms are able to shut down Bcr-Abl activity and result in caspase-dependent cell death in TKIs-resistant and -sensitive Bcr-Abl-positive cell lines. A schematic representation of these findings is presented in Figure 7. Our results Screening Libraries demonstrate that regimens of bortezomib combined with mitotic inhibitors are associated with Bcr-Abl and/or P-BcrAbl downregulation. Few other agents have been shown to induce a significant Bcr-Abl downregulation when used in combination with imatinib. Moreover, the pan-CDK PCI-32765 inhibitor flavopiridol, the heat shock protein 90 antagonist 17-AAG and the histone deacetylase inhibitor SAHA were previously revealed to induce apoptosis in combination with bortezomib, an effect associated with Bcr-Abl downregulation. Although the exact mechanism of Bcr-Abl downregulation is still unclear, it seems plausible that the decrease of Bcr-Abl levels and its inactivation contribute, at least in part, to the caspase-mediated cell death induced by these combinations, including the bortezomib/mitotic inhibitors regimens. Our results point out that a bortezomib/paclitaxel combination inhibits STAT3 and STAT5 activation. Bortezomib/BI 2536 combination similarly results in a decrease in P-STAT5 levels in K562 cells. As previously shown, Bcr-Abl phosphorylates and activates STAT3 and STAT5 transcription factors resulting in cellular survival and proliferation. Constitutive activation of STAT5 is known to be critical for the maintenance of chronic myeloid leukemia and STAT3 is also constitutively active in Bcr-Abl-positive embryonic stem cells. Thus, cell death induced by inhibition of Bcr-Abl with imatinib in Bcr-Abl-positive cells 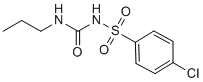 is at least in part related to the inhibition of STAT signaling. Additionally, it is known that JAKSTAT pathway activation contributes to imatinib and nilotinib resistance in Bcr-Abl-positive progenitors. All these findings suggest that STAT3/STAT5 signaling inhibition plays an important role in bortezomib/paclitaxel- or bortezomib/BI 2536-induced cell death, in Bcr-Abl-positive cells. Several pathways are known to be critical downstream mediators of the Bcr-Abl pro-survival and pro-leukemogenic effects. Bcr-Abl is phosphorylated at multiple phosphorylation sites, resulting in binding/phosphorylation of downstream BcrAbl mediators. Phosphorylation of Tyrosine 177 induces the formation of a Lyn – Gab2 – Bcr-Abl complex, important in BcrAbl-induced tumorigenesis. Lyn tyrosine kinase binding to phosphorylated and active Bcr-Abl leads to Lyn activation by phosphorylation. Lyn further regulates survival and responsiveness of CML cells to inhibition of Bcr-Abl kinase. Interestingly, Lyn kinase can also phosphorylate Bcr-Abl, resulting in a potential feedback mechanism. Additionally, Bcr-Abl phosphorylates CrkL adaptor protein, an event needed for Bcr-Abl-induced leukemia. CrkL can enhance cell migration and Bcr-Abl-mediated leukemogenesis. Thus, Lyn and CrkL are key regulators and downstream mediators of BcrAbl-induced survival and leukemogenesis that can be inhibited by downregulation or inhibition of Bcr-Abl. Our results demonstrate that the combined treatment with bortezomib and paclitaxel is able to inhibit the activity of these important BcrAbl downstream mediators. JNK activation was previously associated with apoptosis induced by bortezomib in Bcr-Abl-positive cells and by bortezomib in combination with the pan-CDK inhibitor Flavopiridol in both Bcr-Abl-positive and negative leukemic cells. In addition, several other studies pointed out the role of JNK activation in cell death of Bcr-Abl-positive or -negative cells. Thus, the activation of JNK seen in our results following bortezomib/paclitaxel treatment in Bcr-Abl-positive cells may contribute to cell death.
is at least in part related to the inhibition of STAT signaling. Additionally, it is known that JAKSTAT pathway activation contributes to imatinib and nilotinib resistance in Bcr-Abl-positive progenitors. All these findings suggest that STAT3/STAT5 signaling inhibition plays an important role in bortezomib/paclitaxel- or bortezomib/BI 2536-induced cell death, in Bcr-Abl-positive cells. Several pathways are known to be critical downstream mediators of the Bcr-Abl pro-survival and pro-leukemogenic effects. Bcr-Abl is phosphorylated at multiple phosphorylation sites, resulting in binding/phosphorylation of downstream BcrAbl mediators. Phosphorylation of Tyrosine 177 induces the formation of a Lyn – Gab2 – Bcr-Abl complex, important in BcrAbl-induced tumorigenesis. Lyn tyrosine kinase binding to phosphorylated and active Bcr-Abl leads to Lyn activation by phosphorylation. Lyn further regulates survival and responsiveness of CML cells to inhibition of Bcr-Abl kinase. Interestingly, Lyn kinase can also phosphorylate Bcr-Abl, resulting in a potential feedback mechanism. Additionally, Bcr-Abl phosphorylates CrkL adaptor protein, an event needed for Bcr-Abl-induced leukemia. CrkL can enhance cell migration and Bcr-Abl-mediated leukemogenesis. Thus, Lyn and CrkL are key regulators and downstream mediators of BcrAbl-induced survival and leukemogenesis that can be inhibited by downregulation or inhibition of Bcr-Abl. Our results demonstrate that the combined treatment with bortezomib and paclitaxel is able to inhibit the activity of these important BcrAbl downstream mediators. JNK activation was previously associated with apoptosis induced by bortezomib in Bcr-Abl-positive cells and by bortezomib in combination with the pan-CDK inhibitor Flavopiridol in both Bcr-Abl-positive and negative leukemic cells. In addition, several other studies pointed out the role of JNK activation in cell death of Bcr-Abl-positive or -negative cells. Thus, the activation of JNK seen in our results following bortezomib/paclitaxel treatment in Bcr-Abl-positive cells may contribute to cell death.
Author: targets inhibitor
For JAK2 over Src-family kinases and Flt-3 which are also key mediators in the maturation of lymphocytes
Toaddress this, we evaluated the effect of a structurally distinct JAK2 Afatinib inhibitor with enhanced selectivity over these other Vorinostat HDAC inhibitor signaling molecules. At exposures that resulted incomparable efficacy to MRLB-11055, this inhibitor demonstrated identical reductions in lymphocyte populations. One explanation for these findings is that the reduction in these cell populations is due, at least in part, to inhibition of JAK2 itself, which is consistent with a role of JAK2-dependent cytokines such as IL-12 in lymphocyte development. We have demonstrated that intermittent dosing can attenuate many of 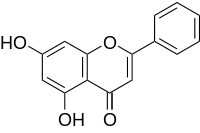 the undesirable effects that will likely be associated with the use of JAK2 inhibitors in the treatment of MPD. In addition to signaling downstream of the EPO receptor, JAK2 plays a role in mediating signaling from a variety of molecules, including IFNc, IL-6, TPO, GM-CSF, prolactin, growth hormone, and angiotensin 1. The JAK2 inhibitor TG101348 has been described as a molecule that is both efficacious in a murine model of PV and sparing of T lymphocytes. While inhibition of pSTAT5 was clearly demonstrated 2 hours after TG101348 administration, it is not clear how prolonged target inhibition was during dosing. As TG101348 required 42 days of continuous treatment to achieve hematocrit reductionsof18%,itisreasonabletopresumethattargetengagement may have been lower relative to MRLB-11055 for a given dosing cycle. Thus the apparently unperturbed T lymphocyte populations maybe explained by a lower level of target engagement. The effect on NK cells, which responded most sensitively to MRLB-11055 inhibition, was not measured with TG101348. We have demonstrated that intermittent dosing of a JAK2 inhibitor can effectively normalize erythroid progenitor populations and thereby effectively treat conditions of polycythemia and splenomegaly in mouse models of PV. Our data can provide signficant guidance to the clinical development of JAK2 inhibitors. While the kinetics of erythropoesis are likely different in human disease, our data provide proof-of-concept for the use of erythroid progenitor populations as early biomarkers of target tissue efficacy, that could guide development of optimized intermittent dosing schemes to provide patients with improved therapy. Furthermore, our data show that lymphoid populations, in particular NK cells, serve as sensitive biomarkers for JAK inhibitor toxicity that is potentially mechanism-based. CD36 is a member of the scavenger receptor family with a broad cell type expression. The specificity of this receptor for oxidized lipoproteins is extensively documented. This receptor is up regulated by ox-LDL in macrophages and contributes to the formation and accumulation of foam cells at sites of arterial lesions during early and late atherosclerosis. This concept was validated by the finding that mice with double CD36 and ApoE deficiency exhibited a greater than 77% decrease in aorta lesions and 50% decrease in aortic sinus lesions despite the induction of a very high atherogenic milieu. This phenomenon was explained by the fact that recruitment and accumulation of foam cells at sites of lesions were considerably reduced in animals lacking CD36. Such a conclusion was however challenged by the observation that combined deficiencies in scavenger A and CD36 functions did not ameliorate atherosclerosis in hyperlipidemic mice. The role of CD36 in the binding and transport of long chain fatty acid in enterocytes and adipocytes is also well documented. The protein is involved in the control of the intestinal transit of cholesterol, triglycerides and fatty acids. CD36 deficiency can also rescue lipotoxic cardiomyopathy and control hepatic triglycerides storage and secretion.
the undesirable effects that will likely be associated with the use of JAK2 inhibitors in the treatment of MPD. In addition to signaling downstream of the EPO receptor, JAK2 plays a role in mediating signaling from a variety of molecules, including IFNc, IL-6, TPO, GM-CSF, prolactin, growth hormone, and angiotensin 1. The JAK2 inhibitor TG101348 has been described as a molecule that is both efficacious in a murine model of PV and sparing of T lymphocytes. While inhibition of pSTAT5 was clearly demonstrated 2 hours after TG101348 administration, it is not clear how prolonged target inhibition was during dosing. As TG101348 required 42 days of continuous treatment to achieve hematocrit reductionsof18%,itisreasonabletopresumethattargetengagement may have been lower relative to MRLB-11055 for a given dosing cycle. Thus the apparently unperturbed T lymphocyte populations maybe explained by a lower level of target engagement. The effect on NK cells, which responded most sensitively to MRLB-11055 inhibition, was not measured with TG101348. We have demonstrated that intermittent dosing of a JAK2 inhibitor can effectively normalize erythroid progenitor populations and thereby effectively treat conditions of polycythemia and splenomegaly in mouse models of PV. Our data can provide signficant guidance to the clinical development of JAK2 inhibitors. While the kinetics of erythropoesis are likely different in human disease, our data provide proof-of-concept for the use of erythroid progenitor populations as early biomarkers of target tissue efficacy, that could guide development of optimized intermittent dosing schemes to provide patients with improved therapy. Furthermore, our data show that lymphoid populations, in particular NK cells, serve as sensitive biomarkers for JAK inhibitor toxicity that is potentially mechanism-based. CD36 is a member of the scavenger receptor family with a broad cell type expression. The specificity of this receptor for oxidized lipoproteins is extensively documented. This receptor is up regulated by ox-LDL in macrophages and contributes to the formation and accumulation of foam cells at sites of arterial lesions during early and late atherosclerosis. This concept was validated by the finding that mice with double CD36 and ApoE deficiency exhibited a greater than 77% decrease in aorta lesions and 50% decrease in aortic sinus lesions despite the induction of a very high atherogenic milieu. This phenomenon was explained by the fact that recruitment and accumulation of foam cells at sites of lesions were considerably reduced in animals lacking CD36. Such a conclusion was however challenged by the observation that combined deficiencies in scavenger A and CD36 functions did not ameliorate atherosclerosis in hyperlipidemic mice. The role of CD36 in the binding and transport of long chain fatty acid in enterocytes and adipocytes is also well documented. The protein is involved in the control of the intestinal transit of cholesterol, triglycerides and fatty acids. CD36 deficiency can also rescue lipotoxic cardiomyopathy and control hepatic triglycerides storage and secretion.
Broad implication in FA membrane transport and may possibly be involved in the metabolic aspects of dyslipidaemia
Observation that CD36 may regulate downstream signalling in enterocytes and stimulate chylomicron synthesis supports this 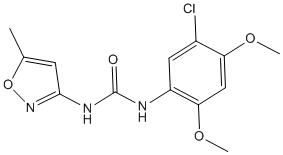 hypothesis. This concept is however questioned by the consistent observation that CD36 gene deletion does not affect plasma TG concentration, LCFA uptake and TG re-esterification in mouse proximal intestine and that postprandial plasma TG concentration is increased in CD36 deficient humans. Therefore, the direct role of CD36 in the intestinal absorption of FA and its pathological hyperlipemia consequence remains an open question. In addition to its potential implication in atherosclerosis and dyslipidaemia, independent studies have suggested that CD36 may also be directly or indirectly involved in diabetes. CD36 deficient humans were reported to have insulin resistance. CD36 gene knock out, however, did not induce insulin resistance in mice. Rather, insulin sensitivity was increased in CD362/2 skeletal muscle. Furthermore, defective insulin signalling was shown to be associated with increased CD36 expression in macrophages. In addition, ox-LDL produced a dramatic reduction of Glyceraldehyde-3-phosphate deshydrogenase in smooth muscle cells resulting in a marked reduction of glucose usage. Together, these observations suggest that CD36 is inversely correlated with insulin sensitivity and plasma lipoproteins. In contrast, animals over expressing CD36 in muscle exhibited decreased plasma concentrations of triglycerides and increased plasma insulin and glucose concentrations and CD36 deficiency induced insulin resistance in the liver of these animals. Therefore, opinions concerning a direct or indirect role of CD36 in insulin resistance and the development of type II GDC-0879 diabetes are diverging. In summary, the preponderance of evidence suggests that CD36 is a central receptor for the detection, accumulation and metabolism of lipids and fatty acids in different cells and tissues. CD36 could then function as a molecular bridge between the development of dyslipidaemia and insulin resistance. If so, it may represent an interesting therapeutic target for the treatment of atherosclerosis, type II diabetes and obesity and their associated cardiovascular diseases. In support with that hypothesis, we show that small molecules with anti-CD36 activity can reduce postprandial hyperlipidaemia and protect against type II diabetes and atherosclerosis. In the present study, correlation between the anti-CD36 Y-27632 inhibitor activity of small molecular weight chemicals and the known pathophysiological activity of this scavenger receptor were established. Although different mechanisms may be involved in the oral versus IP activity of these inhibitors, both administrations were able to improve the metabolic profile of defined and independent rodent models. A significant reduction of the plasma concentration of triglycerides and a better glucose usage were observed at pharmacological doses with a concomitant reduction of the atherosclerotic and diabetic consequences of these attributes. CD36 is a well characterized FA translocase and an oxidized LDL receptor expressed in many cell types including macrophages, adipocytes, endothelial cells and enterocytes. Expression of this gene is ligand-binding dependent and can either be up or down regulated. For instance, ox-LDL-CD36 interaction up regulates a PPARc-dependent CD36 gene expression in monocytes-macrophages whereas interaction with FA down regulates gene expression and protein synthesis in enterocytes, but can up regulate the gene in adipocytes. In addition, CD36 may or may not be associated with companion molecules.
hypothesis. This concept is however questioned by the consistent observation that CD36 gene deletion does not affect plasma TG concentration, LCFA uptake and TG re-esterification in mouse proximal intestine and that postprandial plasma TG concentration is increased in CD36 deficient humans. Therefore, the direct role of CD36 in the intestinal absorption of FA and its pathological hyperlipemia consequence remains an open question. In addition to its potential implication in atherosclerosis and dyslipidaemia, independent studies have suggested that CD36 may also be directly or indirectly involved in diabetes. CD36 deficient humans were reported to have insulin resistance. CD36 gene knock out, however, did not induce insulin resistance in mice. Rather, insulin sensitivity was increased in CD362/2 skeletal muscle. Furthermore, defective insulin signalling was shown to be associated with increased CD36 expression in macrophages. In addition, ox-LDL produced a dramatic reduction of Glyceraldehyde-3-phosphate deshydrogenase in smooth muscle cells resulting in a marked reduction of glucose usage. Together, these observations suggest that CD36 is inversely correlated with insulin sensitivity and plasma lipoproteins. In contrast, animals over expressing CD36 in muscle exhibited decreased plasma concentrations of triglycerides and increased plasma insulin and glucose concentrations and CD36 deficiency induced insulin resistance in the liver of these animals. Therefore, opinions concerning a direct or indirect role of CD36 in insulin resistance and the development of type II GDC-0879 diabetes are diverging. In summary, the preponderance of evidence suggests that CD36 is a central receptor for the detection, accumulation and metabolism of lipids and fatty acids in different cells and tissues. CD36 could then function as a molecular bridge between the development of dyslipidaemia and insulin resistance. If so, it may represent an interesting therapeutic target for the treatment of atherosclerosis, type II diabetes and obesity and their associated cardiovascular diseases. In support with that hypothesis, we show that small molecules with anti-CD36 activity can reduce postprandial hyperlipidaemia and protect against type II diabetes and atherosclerosis. In the present study, correlation between the anti-CD36 Y-27632 inhibitor activity of small molecular weight chemicals and the known pathophysiological activity of this scavenger receptor were established. Although different mechanisms may be involved in the oral versus IP activity of these inhibitors, both administrations were able to improve the metabolic profile of defined and independent rodent models. A significant reduction of the plasma concentration of triglycerides and a better glucose usage were observed at pharmacological doses with a concomitant reduction of the atherosclerotic and diabetic consequences of these attributes. CD36 is a well characterized FA translocase and an oxidized LDL receptor expressed in many cell types including macrophages, adipocytes, endothelial cells and enterocytes. Expression of this gene is ligand-binding dependent and can either be up or down regulated. For instance, ox-LDL-CD36 interaction up regulates a PPARc-dependent CD36 gene expression in monocytes-macrophages whereas interaction with FA down regulates gene expression and protein synthesis in enterocytes, but can up regulate the gene in adipocytes. In addition, CD36 may or may not be associated with companion molecules.
MiR-200c overexpression in apoptosis as compared to cells transfected with scrambled pre-miRs
Noxa protein induction is necessary for cell death to occur following treatment with some cytotoxic cancer drugs, we set out to investigate if Noxa is regulated by microRNAs. Any given gene is generally predicted to be regulated by many different microRNAs. One major obstacle in microRNA research is that the numerous bioinformatic tools available for target prediction invariably give a large set of false positive results. Therefore, we made use of a luciferasebased screening method to pick out the most relevant microRNAs that target Noxa. Cloning the 39UTR of Noxa downstream of a luciferase reporter and introducing this construct into cells allowed us to determine to what degree the reporter activity is repressed in different tissues. This analysis was then complemented with luciferase experiments using deletion constructs that pinpointed the critical regulatory part of the 39UTR. Finally, the combined results were then compared with existing microRNA expression profiling data to identify candidate microRNA that might account for the differential luciferase activity. Using this screening system we identified miR-200c as a new regulator of Noxa. MiR-200c was shown to repress both basal and stressBYL719 induced Noxa protein expression. Surprisingly, enforced miR200c expression at the same time led to increased bortezomibinduced apoptosis. This apparent discrepancy was reconciled by the finding that in cells devoid of Noxa expression, miR-200c caused an even greater increase in apoptosis. These data suggest that miR-200c potentiates apoptosis induced by proteasomal inhibitors but that it concomitantly represses Noxa which leads to an attenuated apoptotic induction. The data in this study define miR-200c as a novel regulator of Noxa and more generally show that microRNA-induced phenotypes must always be viewed as the complex results of a large number of occurring individual microRNA:mRNA target interactions. This clinically used drug was chosen since it has been shown that Noxa induction is important for bortezomib-induced cell death. Treatment of HCT116 cells with clinically relevant doses of bortezomib led to a time- and dose-dependent induction of Noxa protein. As can be seen in Figure 5A, overexpression of miR-200c in HCT116 cells treated with bortezomib led to a downregulation of Noxa at all doses. Surprisingly, at the same time miR-200c overexpression resulted in increased bortezomib-induced apoptosis as assessed by immunoblotting for cleaved caspase 3 and cleaved PARP. In order to directly test how apoptosis induction is affected by miR-200c overexpression, Annexin V/PI staining was performed on HCT116 left untreated or treated with bortezomib. Again, in both cases miR-200c overexpression led to increased cell death, as compared to a scrambled pre-miR control oligonucleotide. A similar result was obtained in the 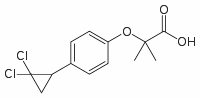 HEK293 cell line. Also, this effect was not restricted to proteasome inhibition, as cells treated with the DNAdamaging drug doxorubicin showed increased apoptosis induction upon miR-200c overexpression as well. Since the effects of miR-200c on Noxa and cell death induced by bortezomib apparently contradict one another, we went on to examine the effect of miR-200c on apoptosis in a setting without Noxa expression. Therefore, we BEZ235 moa knocked down Noxa expression in bortezomib-treated HCT116 cells using siRNA oligos. Knockdown of Noxa led to an expected decrease in both Noxa protein levels and proteasome inhibitor-induced apoptosis as measured by Annexin V/PI staining. Interestingly, when Noxa was knocked down, miR-200c overexpression had an even more pronounced effect on apoptosis induction. Indeed, in cells transfected with control siRNA oligos.
HEK293 cell line. Also, this effect was not restricted to proteasome inhibition, as cells treated with the DNAdamaging drug doxorubicin showed increased apoptosis induction upon miR-200c overexpression as well. Since the effects of miR-200c on Noxa and cell death induced by bortezomib apparently contradict one another, we went on to examine the effect of miR-200c on apoptosis in a setting without Noxa expression. Therefore, we BEZ235 moa knocked down Noxa expression in bortezomib-treated HCT116 cells using siRNA oligos. Knockdown of Noxa led to an expected decrease in both Noxa protein levels and proteasome inhibitor-induced apoptosis as measured by Annexin V/PI staining. Interestingly, when Noxa was knocked down, miR-200c overexpression had an even more pronounced effect on apoptosis induction. Indeed, in cells transfected with control siRNA oligos.
Given the ability of Noxa to fine-tune apoptotic signaling in response to various
Concerning tissue-specific responses, the coleoptile was the only organ which showed an even response to both Pcz and Ucz treatment in Mo20W, A619, and B73. In Torin 1 contrast, Pcz sensitivity in the roots and true leaves ranged from resistant to highly susceptible. The degree of Pcz response in maize roots seems therefore dependent on the genetic Masitinib clinical trial background of the maize line. The data also indicates differential hormonal regulation of tissue growth in aerial organs of maize inbreds. In rice and wheat tissue culture, accumulation of Pcz against a concentration gradient has been reported. This indicates active uptake systems in these grass species. In Monilinia fructicola, the ABC transporter MfABC1 is induced upon Pcz treatment, which suggests a possible role for transporters of the ABC family in Pcz uptake in plants and fungi. Differences in either root uptake, in planta transport, and/or Pcz catabolism may be responsible for the observed variances between maize inbreds. Nonetheless, our results also indicated a relation of Pcz- and BLsensitivity between the inbred lines. Compared  to Mo20W and B73, W22 and A619 plants exhibited a smaller inhibition of root elongation in the presence of either Pcz or higher concentrations of BL. We therefore conclude that the genetic diversity between these maize lines influences their response to BRs. Death induced by the intrinsic mitochondrial pathway is initiated by perturbation of the mitochondrial membrane, and proceeds via release of cytochrome c and other apoptogenic factors from the intermembrane space of this organelle. This process is tightly regulated by the anti- and pro-apoptotic members of the Bcl-2 family. Cytochrome c release in response to various types of cellular stress is suggested to occur via pores formed by homo and hetero-oligomers of the pro-apoptotic Bcl-2 family members Bak and Bax. The actual ratio of anti- to proapoptotic Bcl-2 family members constitutes a sensor and sets the threshold of susceptibility to apoptosis for the cell. That the relative abundance of anti-apoptotic and pro-apoptotic regulators also critically influences tumorigenesis is illustrated by the recurring perturbation of this balance in cancer. Consequently, the expression of Bcl-2 family members is normally tightly regulated at multiple levels including transcriptional activation and proteasomal degradation. In recent years, microRNAs have emerged as important regulators of gene expression. MicroRNAs are 21-23 bp long non-coding RNAs that function mainly through targeting the 39UTR of specific genes and thereby inhibiting the translation of their encoded protein or degrading the target mRNA. With their ability to regulate multiple genes simultaneously, microRNAs have fundamental roles in such diverse processes as proliferation, apoptosis and differentiation. Furthermore, many microRNAs, such as those of the miR-15, let-7, or miR-17 families have been shown to be deregulated in cancer, resulting in the altered expression of target genes important for tumor development. Some Bcl-2 family members have been shown to be regulated by microRNAs, such as Bcl-2, which is regulated by miR-15/16 and miR-148a, and Mcl-1, which is regulated by miR-29. However, for many of the Bcl-2 family members, including the pro-apoptotic p53 target gene Noxa, it is unknown whether microRNA regulation takes place. Like other BH3-only proteins, Noxa has the capacity to bind and neutralize pro-survival Bcl-2 family members. However, it has a restricted binding pattern and mainly interacts with Mcl-1. Among other things, this interaction leads to proteasomal degradation of Mcl-1, which in turn has been shown to be a prerequisite for apoptosis in response to for example UV irradiation.
to Mo20W and B73, W22 and A619 plants exhibited a smaller inhibition of root elongation in the presence of either Pcz or higher concentrations of BL. We therefore conclude that the genetic diversity between these maize lines influences their response to BRs. Death induced by the intrinsic mitochondrial pathway is initiated by perturbation of the mitochondrial membrane, and proceeds via release of cytochrome c and other apoptogenic factors from the intermembrane space of this organelle. This process is tightly regulated by the anti- and pro-apoptotic members of the Bcl-2 family. Cytochrome c release in response to various types of cellular stress is suggested to occur via pores formed by homo and hetero-oligomers of the pro-apoptotic Bcl-2 family members Bak and Bax. The actual ratio of anti- to proapoptotic Bcl-2 family members constitutes a sensor and sets the threshold of susceptibility to apoptosis for the cell. That the relative abundance of anti-apoptotic and pro-apoptotic regulators also critically influences tumorigenesis is illustrated by the recurring perturbation of this balance in cancer. Consequently, the expression of Bcl-2 family members is normally tightly regulated at multiple levels including transcriptional activation and proteasomal degradation. In recent years, microRNAs have emerged as important regulators of gene expression. MicroRNAs are 21-23 bp long non-coding RNAs that function mainly through targeting the 39UTR of specific genes and thereby inhibiting the translation of their encoded protein or degrading the target mRNA. With their ability to regulate multiple genes simultaneously, microRNAs have fundamental roles in such diverse processes as proliferation, apoptosis and differentiation. Furthermore, many microRNAs, such as those of the miR-15, let-7, or miR-17 families have been shown to be deregulated in cancer, resulting in the altered expression of target genes important for tumor development. Some Bcl-2 family members have been shown to be regulated by microRNAs, such as Bcl-2, which is regulated by miR-15/16 and miR-148a, and Mcl-1, which is regulated by miR-29. However, for many of the Bcl-2 family members, including the pro-apoptotic p53 target gene Noxa, it is unknown whether microRNA regulation takes place. Like other BH3-only proteins, Noxa has the capacity to bind and neutralize pro-survival Bcl-2 family members. However, it has a restricted binding pattern and mainly interacts with Mcl-1. Among other things, this interaction leads to proteasomal degradation of Mcl-1, which in turn has been shown to be a prerequisite for apoptosis in response to for example UV irradiation.