Reversine Although SGs are referred to as stalled translation preinitiation complexes, as well as on intensity and type of the stress. For example, SGs induced by a high concentration of ethanol in S. cerevisiae contain only the eIF3c/Nip1 subunit of the eIF3 complex and SGs induced by a prolonged glucose deprivation do not harbor the eIF3 complex at all. With respect to the intensity of the stress, treatment with a low concentration of NaN3 does not affect the distribution of eIF3a but at higher concentration this drug induces eIF3a accumulation in SGs. 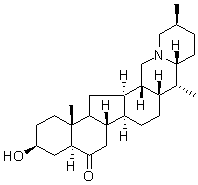 We show here that SGs induced by the robust heat shock in S. cerevisiae contain translation elongation factors eEF3 and eEF1Bc2 together with translation termination factors eRF1 and eRF3. These factors have never been observed in SGs of any other eukaryotic cell. However, the termination factors have been found to accumulate in P-bodies. Those authors have concluded that presence of translation termination factors in Pbodies is BAY-60-7550 clinical trial coupled to the P-bodies assembly. A similar role could be suggested for presence of these factors in heat-induced SGs. The proteins with self-aggregation domain, like TIA-1 or TIAR in mammalian cells, have been described to influence dynamics of SGs. A newly identified component of the heat-induced SGs in S.cerevisiae, Sup35p, possesses a prion-like domain at the N-terminus. Sup35p can thus convert into the prion form, known as. The N-terminal part of the protein is indispensable for the prion formation and maintenance. Similarly to a situation in mammalian cells we found that rather a non-prion part of Sup35p is responsible for accumulation of the protein in SGs. However, observations that SGs are formed even in the absence of the N-terminal prion-like domain of Sup35p indicate that unlike in mammals, the assembly of heatinduced SGs in S. cerevisiae is not driven by these “prion” structural elements. This hypothesis is also supported by our earlier findings that heat-induced SGs are formed even in the absence of yeast orthologs of mammalian TIA-1 and TIAR proteins, Ngr1 and Pub1 proteins in S. cerevisiae. Translation termination factors eRF1 and eRF3 are responsible for effective termination of translation. In addition, they seem to be required for an effective function of the fungal-specific elongation factor eEF3 in recycling of the translation posttermination complexes after the release of newly synthetized peptide chains. In this respect, identification of elongation and termination factors in heatinduced SGs may indicate that these SGs are composed of translation posttermination complexes stalled before the ribosome recycling step. However, we did not observe any accumulation of several essential proteins of the 60S ribosomal subunits under robust heat shock and Grousl et al.. In addition, all the published information on recycling of the translation posttermination complexes comes from in vitro experiments only. Therefore, it is currently unclear, how recycling is catalyzed in vivo and the reasons for presence of the translation elongation and the termination factors in robust heat shockinduced SGs remain to be elucidated. Whereas different roles for SGs and P-bodies in cell survival upon heat stress conditions could be suggested, both accumulations are always closely spatially and functionally intertwined. In S. cerevisiae cells, P-bodies promote formation of SGs.
We show here that SGs induced by the robust heat shock in S. cerevisiae contain translation elongation factors eEF3 and eEF1Bc2 together with translation termination factors eRF1 and eRF3. These factors have never been observed in SGs of any other eukaryotic cell. However, the termination factors have been found to accumulate in P-bodies. Those authors have concluded that presence of translation termination factors in Pbodies is BAY-60-7550 clinical trial coupled to the P-bodies assembly. A similar role could be suggested for presence of these factors in heat-induced SGs. The proteins with self-aggregation domain, like TIA-1 or TIAR in mammalian cells, have been described to influence dynamics of SGs. A newly identified component of the heat-induced SGs in S.cerevisiae, Sup35p, possesses a prion-like domain at the N-terminus. Sup35p can thus convert into the prion form, known as. The N-terminal part of the protein is indispensable for the prion formation and maintenance. Similarly to a situation in mammalian cells we found that rather a non-prion part of Sup35p is responsible for accumulation of the protein in SGs. However, observations that SGs are formed even in the absence of the N-terminal prion-like domain of Sup35p indicate that unlike in mammals, the assembly of heatinduced SGs in S. cerevisiae is not driven by these “prion” structural elements. This hypothesis is also supported by our earlier findings that heat-induced SGs are formed even in the absence of yeast orthologs of mammalian TIA-1 and TIAR proteins, Ngr1 and Pub1 proteins in S. cerevisiae. Translation termination factors eRF1 and eRF3 are responsible for effective termination of translation. In addition, they seem to be required for an effective function of the fungal-specific elongation factor eEF3 in recycling of the translation posttermination complexes after the release of newly synthetized peptide chains. In this respect, identification of elongation and termination factors in heatinduced SGs may indicate that these SGs are composed of translation posttermination complexes stalled before the ribosome recycling step. However, we did not observe any accumulation of several essential proteins of the 60S ribosomal subunits under robust heat shock and Grousl et al.. In addition, all the published information on recycling of the translation posttermination complexes comes from in vitro experiments only. Therefore, it is currently unclear, how recycling is catalyzed in vivo and the reasons for presence of the translation elongation and the termination factors in robust heat shockinduced SGs remain to be elucidated. Whereas different roles for SGs and P-bodies in cell survival upon heat stress conditions could be suggested, both accumulations are always closely spatially and functionally intertwined. In S. cerevisiae cells, P-bodies promote formation of SGs.