The retained pre-bound peptide and the position of the exchange peptide favoring the latter’s access to the groove. In either scenario, DM would promote the folding of the peptide/MHCII complex to the final conformation. In a stochastic competition, both peptides would simultaneously attempt 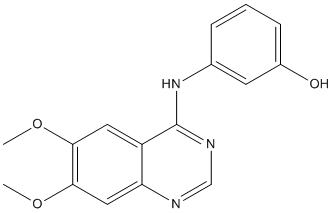 to fit into the groove, and half of these events would result in rebinding of the original peptide when the two peptides show the same affinity. In an ordered model, the exchange peptide would be tested first and only if it was incapable of binding would the prebound peptide return to the groove. Although we are pursuing experiments to discriminate between these possibilities, we do observe a difference in the cooperativity of binding in the presence of the prebound peptide vs. that observed in the absence of the prebound peptide using largely empty soluble DR1 molecules. One explanation for this difference would be that the by forming an initial complex, the prebound peptide shifts the peptide/MHCII complex into a conformer receptive to subsequent efficient cooperative folding in the presence of a competitor peptide. In vivo, the CLIP peptide may play a similar role. We should point out that the selection of which peptide will fold into the MHCII is restricted to the two peptides in the complex. The MHCII is unavailable to third party peptides, as the experiments shown in Figures 1a�Cb, as well as Figure 3c are performed Benzoylaconine adding a large excess of exchange peptide and clearly no mass action effect can be detected for peptides with intrinsic low affinity for MHCII. Mapping the location of the exchange peptide on the peptide/ MHCII complex in the presence or absence of DM will be an important step in refining the mechanism. Due to the need for diverse competitor peptide recognition, the most likely possibility is that the incoming competitor peptide may associate with the exchanging complex by forming partial hydrogen bond or hydrophobic interactions with the destabilized peptide binding groove. As the amino acid polymorphism in the peptide binding groove Ginsenoside-F5 across different MHCII alleles result in “anchor-pocket” interactions of varying strength, we expect that hydrogen bonding may provide the majority of the binding energy for competitor peptide recognition. However, we cannot entirely exclude the possibility that the competitor peptide interacts with a distinct site present across MHCII alleles.
to fit into the groove, and half of these events would result in rebinding of the original peptide when the two peptides show the same affinity. In an ordered model, the exchange peptide would be tested first and only if it was incapable of binding would the prebound peptide return to the groove. Although we are pursuing experiments to discriminate between these possibilities, we do observe a difference in the cooperativity of binding in the presence of the prebound peptide vs. that observed in the absence of the prebound peptide using largely empty soluble DR1 molecules. One explanation for this difference would be that the by forming an initial complex, the prebound peptide shifts the peptide/MHCII complex into a conformer receptive to subsequent efficient cooperative folding in the presence of a competitor peptide. In vivo, the CLIP peptide may play a similar role. We should point out that the selection of which peptide will fold into the MHCII is restricted to the two peptides in the complex. The MHCII is unavailable to third party peptides, as the experiments shown in Figures 1a�Cb, as well as Figure 3c are performed Benzoylaconine adding a large excess of exchange peptide and clearly no mass action effect can be detected for peptides with intrinsic low affinity for MHCII. Mapping the location of the exchange peptide on the peptide/ MHCII complex in the presence or absence of DM will be an important step in refining the mechanism. Due to the need for diverse competitor peptide recognition, the most likely possibility is that the incoming competitor peptide may associate with the exchanging complex by forming partial hydrogen bond or hydrophobic interactions with the destabilized peptide binding groove. As the amino acid polymorphism in the peptide binding groove Ginsenoside-F5 across different MHCII alleles result in “anchor-pocket” interactions of varying strength, we expect that hydrogen bonding may provide the majority of the binding energy for competitor peptide recognition. However, we cannot entirely exclude the possibility that the competitor peptide interacts with a distinct site present across MHCII alleles.