Bioinformatic analysis was used to Praeruptorin-B identify a number of processes affected by DENV-2 infection. The results of the SILAC-MS analysis was validated for seven selected proteins by Western blo ing and immunofluorescence microscopy. This is the first report of the application of SILACMS 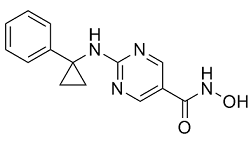 to study changes in the host cell proteome in response to DENV infection and demonstrates the power of this technique for identifying and quantifying changes in cellular protein amounts in response to DENV infection. In order to conduct the proteomic analysis, human lung carcinoma A549 cells were grown for eight cell doublings in either light or heavy Senegenin labeled media before being mock infected or infected with DENV-2 respectively. Although not believed to represent a target cell for DENV in vivo, A549 cells are highly permissive for DENV infection and have been used in previous studies examining the effect of DENV on the cellular innate immune response and the host cell transcriptome. At 28 hours p.i., a time point determined by growth curve analysis to lie in exponential phase of DENV replication, the cells were harvested and fractionated into nuclear and cytoplasmic extracts. At this time there was no obvious difference in the morphology of the mock and DENV infected cells, suggesting that there was li le cell death. IFA analysis of a sample of the mock and infected A549 cells revealed that 100% of the cells had been infected. The fractionation was done to reduce the overall complexity of the sample and to identify proteins that were altered in amount or redistributed between the cytoplasm and nucleus during DENV infection. DENV is known to replicate in tight association with perinuclear membranes therefore the cells were fractionated using a procedure that removed as much of the perinuclear membrane as possible without disrupting the nuclei. The presence of protein markers in the cellular fractions specific to the nucleus, cytoplasm and DENV infection were analyzed by Western blo ing to validate the infection and fractionation procedures. The analysis showed that whilst the nuclear and soluble cytoplasmic protein fractions were distinct, the lack of detergent in the lysis buffer led to the presence of some membraneous/ cytoskeletal proteins in the nuclear fractions. It appeared that the perinuclear membrane and the associated viral replication structures were not totally removed from the nuclei, as evidenced by a minor amount of the virus E protein, which is cytoplasmically localized, in the nuclear fraction. By contrast, the NS5 protein is known to be found in both the nucleus and cytoplasm of infected cells. Equal protein amounts from the cytoplasmic fractions of the mock and DENV-2 infected cells were pooled and the same procedure repeated for the nuclear fractions. The proteins in the cytoplasmic and nuclear fractions were separated by 1D SDSPAGE, subject to in-gel tryptic digestion and the peptides analyzed by quantitative LC-MS/MS to determine the relative amounts of proteins in the nuclear and cytoplasmic fractions from mock and DENV-2 infected cells. This procedure was done once and resulted in the identification of 4053 and 2881 cellular proteins in the cytoplasmic and nuclear fractions of which 3098 and 2115 respectively, were reliably quantified.
to study changes in the host cell proteome in response to DENV infection and demonstrates the power of this technique for identifying and quantifying changes in cellular protein amounts in response to DENV infection. In order to conduct the proteomic analysis, human lung carcinoma A549 cells were grown for eight cell doublings in either light or heavy Senegenin labeled media before being mock infected or infected with DENV-2 respectively. Although not believed to represent a target cell for DENV in vivo, A549 cells are highly permissive for DENV infection and have been used in previous studies examining the effect of DENV on the cellular innate immune response and the host cell transcriptome. At 28 hours p.i., a time point determined by growth curve analysis to lie in exponential phase of DENV replication, the cells were harvested and fractionated into nuclear and cytoplasmic extracts. At this time there was no obvious difference in the morphology of the mock and DENV infected cells, suggesting that there was li le cell death. IFA analysis of a sample of the mock and infected A549 cells revealed that 100% of the cells had been infected. The fractionation was done to reduce the overall complexity of the sample and to identify proteins that were altered in amount or redistributed between the cytoplasm and nucleus during DENV infection. DENV is known to replicate in tight association with perinuclear membranes therefore the cells were fractionated using a procedure that removed as much of the perinuclear membrane as possible without disrupting the nuclei. The presence of protein markers in the cellular fractions specific to the nucleus, cytoplasm and DENV infection were analyzed by Western blo ing to validate the infection and fractionation procedures. The analysis showed that whilst the nuclear and soluble cytoplasmic protein fractions were distinct, the lack of detergent in the lysis buffer led to the presence of some membraneous/ cytoskeletal proteins in the nuclear fractions. It appeared that the perinuclear membrane and the associated viral replication structures were not totally removed from the nuclei, as evidenced by a minor amount of the virus E protein, which is cytoplasmically localized, in the nuclear fraction. By contrast, the NS5 protein is known to be found in both the nucleus and cytoplasm of infected cells. Equal protein amounts from the cytoplasmic fractions of the mock and DENV-2 infected cells were pooled and the same procedure repeated for the nuclear fractions. The proteins in the cytoplasmic and nuclear fractions were separated by 1D SDSPAGE, subject to in-gel tryptic digestion and the peptides analyzed by quantitative LC-MS/MS to determine the relative amounts of proteins in the nuclear and cytoplasmic fractions from mock and DENV-2 infected cells. This procedure was done once and resulted in the identification of 4053 and 2881 cellular proteins in the cytoplasmic and nuclear fractions of which 3098 and 2115 respectively, were reliably quantified.