Most importantly, the rs6020 G-to-A polymorphism was highly prevalent in our Chinese population, but it is 0% in the Western population. The presence of the rs6020 G-to-A polymorphism predicted a high chance of coagulation Danshensu abnormalities in patients with ONFH. The marine bacterium Vibrio fischeri lives symbiotically in light organs of some fishes and squids, and exhibits bioluminescence at high population densities. Nealson et al. CAY10505 reported that this bioluminescence was due to the accumulation of an autoinducer, and that the intensity of bioluminescence positively correlated with bacterial population density. This phenomenon is called “quorum sensing”, and considerable attention has been paid to determine the signaling molecules involved in it. N-3oxohexanoyl-L-homoserine lactone, a type of acyl-homoserine lactone, was the first identified molecule in the process. Interestingly, AHL was found to freely diffuse through cell membranes and to play a role in cell�Ccell communication. As reported, at low bacterial population density, the concentration of AHLs remains low; however, their concentration increases with an increase in bacterial population density. At or above a particular threshold, they effectively bind to LuxR, which has been identified as an intracellular regulatory protein that mediates the formation of the LuxR:AHL complex. This dimer complex subsequently binds to the promoter of lux box and induces the expression of downstream genes such as luxC, luxD, luxA, luxB and luxE, which generates bioluminescence. V. fischeri lux box contains the promoters lux pL and lux pR that 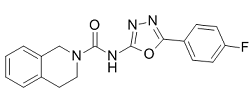 regulate the transcription of luxR and luxI, respectively. luxI encodes a synthase, LuxI, which produces signaling molecule AHLs. luxI expression is upregulated by the interaction between the LuxR:AHL complex and lux box. Therefore, a positive feedback is involved in sensing signals. In brief, quorum sensing in bacterial bioluminescence is tightly controlled by several key elements such as lux box, luxR, and luxI. This provides the basis for building an artificial quorum-sensing circuit. You et al. reported a programmed cell-death circuit by cloning luxR and luxI under the control of a synthetic promoter Plac/ara-1, and cotransforming the bacterial lethal gene ccdB into Escherichia coli cells. These engineered bacteria could successfully produce and release autoinducer AHLs, which mediated luxR and ccdB expression in a feedback manner. As these engineered bacteria grew, they continuously released autoinducer AHLs into their environment. When AHL concentration reached a particular threshold, AHLs combined with LuxR and activated ccdB expression to produce CcdB. Consequently, CcdB poisoned bacterial gyrase and resulted in the death of numerous bacterial cells. However, when bacterial population density decreased, the concentration of LuxR:AHL dimer complexes required to activate lux pR was insufficient, and resulted in termination of cell death. Thereafter, the cells entered into a new round of growth. Once population density reached the abovementioned threshold, another round of cell death was triggered. This system successfully mimicked the situation of quorum sensing, in which bacterial cell growth and death could be regulated through bacterial population density. However, this system was cotransformed using two separate plasmids under the control the promoters Plac/ara-1 and PluxI. This could be problematic because of an imbalance in the copy numbers of the two plasmids, making it difficult to accurately investigate cellular communication on a genetic basis.
regulate the transcription of luxR and luxI, respectively. luxI encodes a synthase, LuxI, which produces signaling molecule AHLs. luxI expression is upregulated by the interaction between the LuxR:AHL complex and lux box. Therefore, a positive feedback is involved in sensing signals. In brief, quorum sensing in bacterial bioluminescence is tightly controlled by several key elements such as lux box, luxR, and luxI. This provides the basis for building an artificial quorum-sensing circuit. You et al. reported a programmed cell-death circuit by cloning luxR and luxI under the control of a synthetic promoter Plac/ara-1, and cotransforming the bacterial lethal gene ccdB into Escherichia coli cells. These engineered bacteria could successfully produce and release autoinducer AHLs, which mediated luxR and ccdB expression in a feedback manner. As these engineered bacteria grew, they continuously released autoinducer AHLs into their environment. When AHL concentration reached a particular threshold, AHLs combined with LuxR and activated ccdB expression to produce CcdB. Consequently, CcdB poisoned bacterial gyrase and resulted in the death of numerous bacterial cells. However, when bacterial population density decreased, the concentration of LuxR:AHL dimer complexes required to activate lux pR was insufficient, and resulted in termination of cell death. Thereafter, the cells entered into a new round of growth. Once population density reached the abovementioned threshold, another round of cell death was triggered. This system successfully mimicked the situation of quorum sensing, in which bacterial cell growth and death could be regulated through bacterial population density. However, this system was cotransformed using two separate plasmids under the control the promoters Plac/ara-1 and PluxI. This could be problematic because of an imbalance in the copy numbers of the two plasmids, making it difficult to accurately investigate cellular communication on a genetic basis.