The metabotropic Coptisine-chloride glutamate receptor family, and the kainate/AMPA-type ionotropic glutamate receptor, are down-regulated in retinas from SIRT6 KO mice. Synaptic transmission between light-excited rod photoreceptors and downstream ON-bipolar neurons is indispensable for dim vision in the mammalian retina. Indeed, disruption of this process leads to congenital stationary night blindness in human patients. Notably, among the metabotropic receptors analyzed, Grm6 was found to be the most significantly down-regulated in KO retinas. Once released from the pre-synapsis, glutamate can bind to different glutamate receptors on the post-synapsis or can be removed from the synaptic cleft by high affinity glutamate transporters located on adjacent neurons and surrounding glial cells to prevent cell death. The major pathway of glutamate metabolism consists of glutamate uptake by glutamate transporters followed by enzymatic conversion of glutamate to non-toxic glutamine by glutamine synthetase. When a glutamate transporter is pharmacologically blocked, inner retinal neurons are exposed to a higher amount of endogenous glutamate, resulting in severe excitotoxic degeneration. These observations suggest that glutamate is neurotoxic when the uptake system is impaired rather than when the release is excessive. Alternatively, impaired expression of a post-synaptic receptor may contribute to the accumulation of the neurotransmitter in the inter-synaptic space. In this context, down-regulation of Grm6 could account for the increment of glutamate in the inter-synaptic space exerting a toxic effect that could explain the increase of TUNEL positive cellsfound in the inner nuclear layer of SIRT6 KO retinas. Since both bipolar and Mu��ller cells are involved in the generation of the b-wave, apoptotic cells in the inner nuclear layer would explain the alteration observed in the ERG. Overall, our studies demonstrated that SIRT6 deficiency causes major chromatin changes in the retina, which is accompanied by marked changes in expression of metabolic genes and metabotropic receptors, likely explaining the severe functional impairment observed in the SIRT6-KO retinas. Previous studies established sirtuins as critical Ascomycin modulators of metabolism, protecting against metabolic- and age-related diseases, such as diabetes, metabolic syndrome, cancer and neurodegenerative disorders. Our results 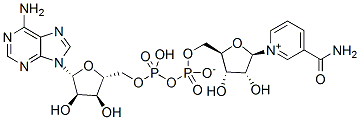 indicate that SIRT6 plays an important role in maintaining normal retinal function. Altered methylation patterns and histone modifications have been identified in different ocular diseases like diabetic retinopathy, glaucoma and age-related macular degeneration. However, a comprehensively characterized epigenomic signature for any ocular disease remains elusive. Several questions arise: is there any epigenetic mark that would predict the onset or the progression of an ocular disease? Do epigenetic factors regulate other cellular pathwaysthat may alter visual function? Although epigenetic therapies have proven to be effective in cancer applications, the benefits of these approaches have not yet been applied for human ophthalmic diseases. Thus far, there is no structural information available for any protein component of the MtrCDE tripartite complex system. However, it has been reported that individual protein components of this tripartite system are able to interact with each other, suggesting that the tripartite MtrCDE pump is assembled in the form of MtrD3-MtrC6-MtrE3.
indicate that SIRT6 plays an important role in maintaining normal retinal function. Altered methylation patterns and histone modifications have been identified in different ocular diseases like diabetic retinopathy, glaucoma and age-related macular degeneration. However, a comprehensively characterized epigenomic signature for any ocular disease remains elusive. Several questions arise: is there any epigenetic mark that would predict the onset or the progression of an ocular disease? Do epigenetic factors regulate other cellular pathwaysthat may alter visual function? Although epigenetic therapies have proven to be effective in cancer applications, the benefits of these approaches have not yet been applied for human ophthalmic diseases. Thus far, there is no structural information available for any protein component of the MtrCDE tripartite complex system. However, it has been reported that individual protein components of this tripartite system are able to interact with each other, suggesting that the tripartite MtrCDE pump is assembled in the form of MtrD3-MtrC6-MtrE3.